

At BilizBio, we are committed to revolutionizing the future of healthcare through cutting-edge biotechnological innovations. Our focus is on developing transformative therapies that address unmet medical needs in areas of high global impact. With a robust pipeline of novel biologics and a relentless pursuit of scientific excellence, BilizBio is at the forefront of discovering and delivering next-generation solutions for complex diseases.
1st
First-mover advantage in treating neurological diseases intranasally
$5.3B
Market opportunity for initial treatment indication in Parkinson's Disease
+$4.0B
Market opportunity targeting additional formulations + drug-device combinations
Intranasal Platform Solution for Neurological Diseases
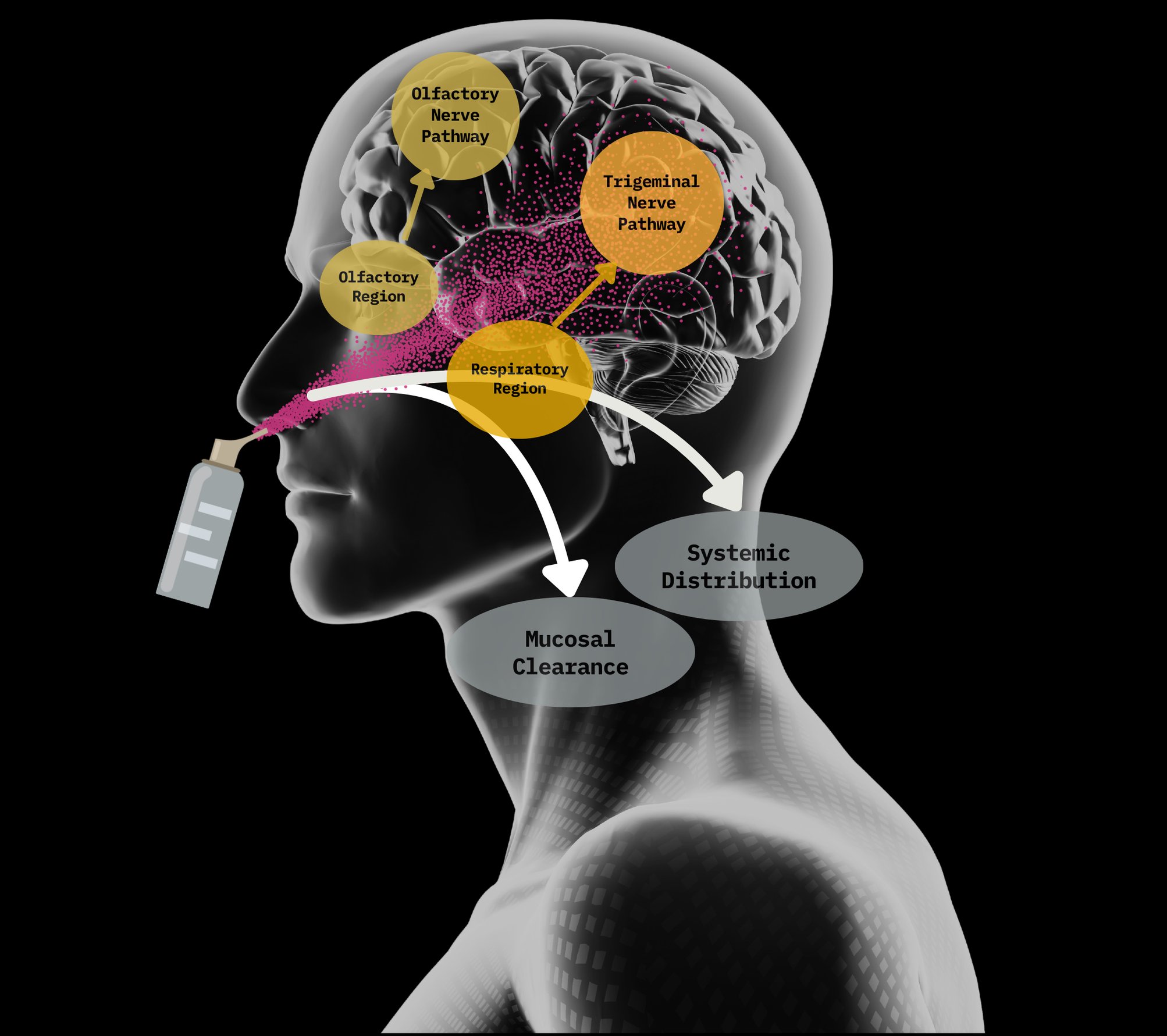
Proprietary airflow system delivers high-velocity, rifled drug dispersion for precise nasal targeting
Enables direct and rapid drug transport to the brain within minutes via neural and vascular pathways
Temperature-stable spray-dried powders enhance absorption through the nasal lining for greater brain exposure and optimizing neurological efficacy and ensure drug is active even in extreme environments